|
|
Materials of this chapter are published in articles:
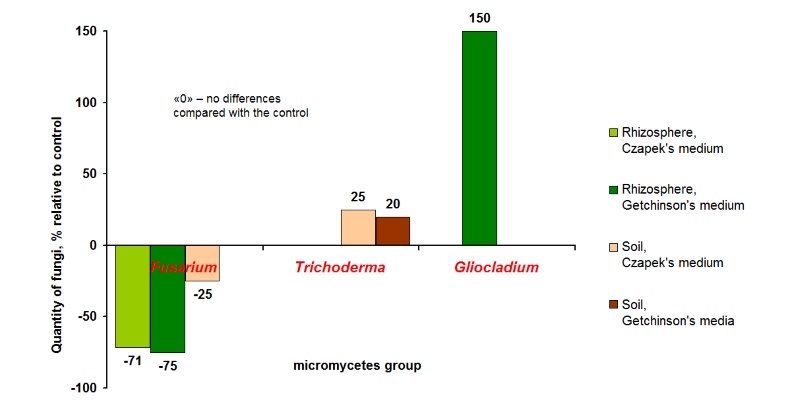
It is difficult to overestimate the role of soil – the main source of fertility – for plant cultivation. However, mindless tillage and also intensive application of chemical pesticides destroy the natural soil microflora. For example, according to recent phytopathological research of one of the well-known advanced farms in the south of Russia, 80-90% of pathogenic microflora were found in soil, and only 10% – positive microflora [Pugachev, 2016]. Especially disastrous situation was observed due to development of Fusarium and root rots – “diseases of intensification”. A large amount of crop residues remains on the fields after harvesting. On the one hand, it is a storehouse of mineral substances and effective method for increasing of soil fertility; on the other hand, it is a source of numerous pathogens of plants. Disturbing of soil microbial community occurs during application of resource-saving technologies – minimal tillage or zero tillage (no-till), which are increasingly gaining popularity. These technologies are usually accompanied by dramatic increase of pesticide application. Pesticides suppress the natural beneficial microflora. As a result, bacterial root rots appear in the soil [Laromenskaya, 1989; Izhevskii, 2006; Kharchenko, 2012]. Natural microbial activity of the soil is decreased by 30-50% under influence of chemical pesticides [Karpun, 2014]. One of the most striking examples is negative influence of glyphosate on soil microorganisms. This herbicide is often used in no-till technology [Daouk, 2013]. Glyphosate promotes the development of pathogens of root rots: genera Gaeumannomyces, Pythium and Fusarium. Widely used fungicides based on tebuconazole completely suppress the growth of beneficial fungi genus Trichoderma in the rhizosphere. This leads to the development of a variety infections in the soil [Zhaliyeva, 2008]. Stubble remains during application of minimal technologies are not plowed and practically are not decomposed. As a result, the basis of farming – soil is turning into bottomless reservoir of infections with which we unsuccessfully fight by applying more and more pesticides. For solving the problems related to increased amount of pathogenic microogranisms in soil after pesticide application, as well as accumulation of pathogenic microorganisms on stubble remains, it is recommended to apply products that are capable to reduce the infection in the soil. According to many data of conducted field trials, Albit makes soil healthier and increases soil fertility. Unlike many analogues, Albit influences not only on plants, but also indirectly stimulates their growth by influencing directionally on microbial community of plant roots and soil. It leads to decrease the number of soil phytopathogens, enhanced supply of plants with nutrients, increase the efficacy of using of nutrients from fertilizers.In comparison with other habitats, soil has more diversity and abundance of microorganisms. More than one billion of live microorganisms are in gram of healthy fertile soil [Bab’eva, Zenova, 1989]. It is a huge strength, which has the significant effect on growth and productivity of agricultural plants. It is only required to «direct» the development of soil microorganisms into «favorable direction» for agrosenosis. Figuratively speaking, billons of soil microorganisms will work for you (supplying the plants with nutrients and protecting them against phytopathogens) or “against you”. Microscopic fungi, some microalgae and a huge diversity of bacteria, such as of genera Bacillus, Pseudomonas, Klebsiella, Azotobacter, Beijerinckia, Clostridium, Arthrobacter, Flavobacterium, Aquaspirillum, Cellulomonas, Cytophaga, Mycobacterium, Derxia, Nocardia, Agromyces, Rhizobium, Agrobacterium and others refer to soil microorganisms. The majority of soil microorganisms positively affect plants [Lysak et al., 2003]. The richest biodiversity and largest number of microorganisms are typical for rhizosphere (the narrow area adjacent to plant roots). The most important factor determining the distinctions of rhizosphere and others parts of soil is the close interaction between microorganisms and plant. Plant activity determines to a great extent gaseous and water metabolism, as well as feed conditions inside of rhizosphere. In turn, soil microorganisms significantly positively affect plants: provide physiologically active compounds, vitamins, fixed nitrogen (nitrogen fixation), release phosphorous, potassium and microelements from soil minerals [Bab’eva, Zenova, 1989]. The majority of known soil bacteria are not free-living, but live in various types of interaction (association, symbiosis, and parasitism) with plants, animals, and fungi. Interaction between bacteria plays the most important part in phyllosphere, rhizoplane, and rhizosphere. In soil community, bacterial species closely interact with each other and with variable environment. Enrichment of soil by organic fertilizer can selectively guide the development of microbial consortiums towards the formation of new associations characterized by other functions [Fukui, 2003]. This approach creates the background of microbiological control of plant pathogens. In the absence of external stress, natural bacterial consortium is a stabilizing factor preventing plant pathogens development within the soil. It is well-known that soil microorganisms can enhance or diminish fungicide effect. Suppressive soils are the soils which resist plant diseases [Singleton, Sainsbury, 1993]. Positive impact of Albit upon plants could be in part explained by indirect effect on soil community. Albit gets into soil mainly from the treated seeds and involve changes in operation of soil microflora including microorganisms in rhizosphere. External influence of sufficient intensity causes microbial succession. Ecological succession is the systematic and well-organized process of change in the biodiversity and species structure of an microbiological community over time. From a practical point of view, it is important to guide microbial succession in the right direction — toward the maximal stimulation of plant growth and suppression of pathogens. Investigations showed that Albit acts within the specified paradigm. Effect of Albit on soil microorganisms was studied at the Pedology Faculty of Moscow State University (the Department of Soil Biology and Agricultural Chemistry). Soil samples from the vegetation experiment pledged at the Department of Agricultural Chemistry in 1999 were studied. It was found that treatment by Albit causes changes in the microbial community of plants’ rhizosphere, resulting in the reduction of pathogenic microscopic fungi number (e.g. Fusarium genus), and increasing of bacterial amount. Also the growth of micromycetes abundance (Gliocladium, Sladosporium and Trichoderma), antagonists of plant pathogens, was shown. After Albit treatment the number of Trichoderma and other soil fungi-antagonists increases. Thus, Albit application is an important factor of biostimulant activity together with plant immunization (Table 1, Fig.1). The influence of Albit on biodiversity of different philogenetic groups of microscopic fungi in the rhizosphere of spring barley (Pot experiment was conducted at the Department of Pedology, Moscow State University, 1999) The table shows the decline in the biodiversity of fungi or increase relative to control (%) after the standard Albit application (seed treatment + sprayings). "0" - no changes compared with the control
** – Microscopic fungi of this philogenetic group were not determined.
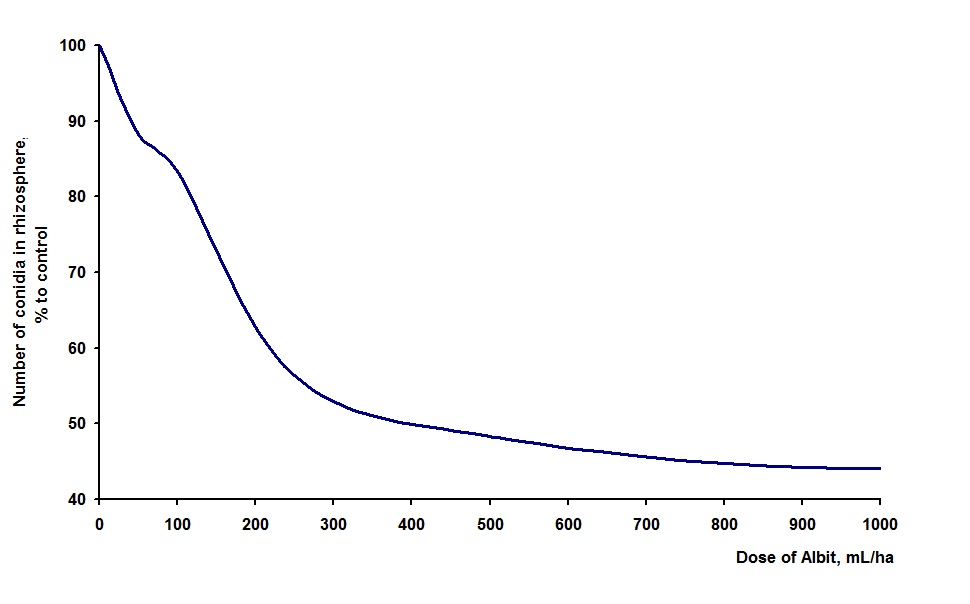
The pot experiments were confirmed afield. According to the Ryazan and Saratov regional plant protection stations, systematical application of Albit improves the phytosanitary conditions of the soil. The experiments conducted in All-Russian Institute of Floriculture and Subtropical Crops demonstrated that Albit application the number of conidia of pathogen Phytophtora cactorum in garden soil was reduced by 52-56% (Fig. 2).
On sugar beet, field trials were conducted in the conditions of the steppe zone of Bashkortostan Republic (Chimshiagroinvest LLC, 2009–2010) [Pusenkova et al., 2016]. 40 mL/hà of Albit were applied twice in a tank mix with herbicides: at the stages of 2-3 true leaves and4-6 true leaves. At least 14 pathogenic species of microfungi were found in soil when the sugar beet was sowed. Species of Penicillium (5 species), Aspergillus (5 species) and Fusarium (2 species) microfungi dominated in rhizosphere. Penicillium aurantiogriseum, Alternaria tenuis, Aspergillus niger are cause agents of sugar beet root rot, and other 5 species – Penicillium glabrum, Fusarium solani var. agrillaceum, Fusarium oxysporum, Aspergillus parvulus, Rhizopus microsporus – enhance the development of root diseases that cause Botrytis-induced grey mould. Treating plants with bioproducts changed species' composition of microfungi in the sugar beet rhizosphere. After a single Albit application, the microfungi composition in the rhizosphere was reduced to 8 species, with Penicillium being the only fungus pathogenic for sugar beet. Development of pathogenic microfungi such as Alternaria tenuis, Aspergillus niger, Aspergillus parvulus, Fusarium oxysporum, and Fusarium solani was suppressed. At the same rate, the number of pathogenic fungi reduced to 8.1% (63.8% in control). Albit nearly eliminated cause agents of sugar beet root rot in the rhizosphere.
While suppressing pathogens, Albit has a stimulating effect on the development of beneficial saprophytic microorganisms. In the experiments of the Agrochemistry department of Moscow State University on barley, Albit treatments increased the total amount of microorganisms in soil and on roots. They also increased the saprophyte and nitrogen-fixing bacteria content in the rhizosphere. Albit increased the total amount of microorganisms grown on the culture media (from 3 to 3.5 million/g in soil and from 8 to 14.7 million on the roots).The amount was noted to increase to a greater extent directly on the roots than in the rhizosphere (Table 2). This effect was attenuated towards the later stages of the growing period. The increase in the number of beneficial microorganisms in soil is the result of both Albit's direct action on soil microbiome and its stimulation of plant growth. Plants treated with Albit produce more root exudates, which leads to the rapid development of soil microflora. This effect can be observed in the photograph from the Keshan farm in the Edirne region (Turkey, 2015): the roots of wheat treated with Albit formed a "protective coat" from the soil bound by root exudates (Fig. 3). Even under drought conditions, a 2 t/ha increase in yield was obtained as a result of the robust development of the root system and the rhizosphere. Table 2. Microbiological characteristics of the soil following the treatment of barley with Albit (based on the pot experiment at Moscow State University, 1999) The number of microorganisms (million of colonies / g of soil), inoculation on glucose-peptone agar media , registered at the tillering stage.
Fig. 3. Rhizosphere microbiocenosis forming intensively on the roots of winter wheat var. Hamza as a result of Albit treatment
The number of growth-stimulating and nitrogen-fixing bacteria in soil increased under the influence of Albit (e.g., Azotobacter), growth-stimulating capacity of the soil increased by 50-100%, its overall toxicity significantly reduced: from 25-55 to 0-30 units (Table 3). Under the influence of Albit the increase in activity of beneficial microorganisms that encourage plant growth, and reduced activity of pathogenic microorganisms was established [Kostina, Zlotnikov, 2000]. Table 3. The influence of treatment of barley with Albit (30 mL/t) on toxicity of rhizosphere soil in pot experiment (Soil Biology Department of Moscow State University, 1999)
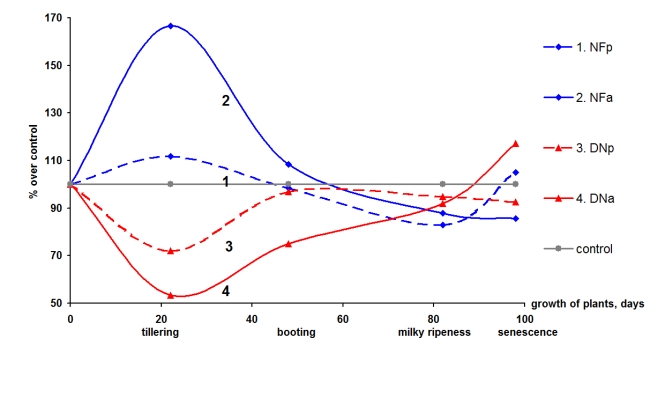
Thus, reorganization of soil microbial community is an important mechanism that reduces the harmfulness of pathogens without inoculation of living biofungicides. Albit just stimulates the growth of fungicidal microorganisms, which are already present in the rhizosphere. Albit does not contain living nitrogen-fixing bacteria. However, Albit enhances the nitrogen fixation activity in rhizosphere due to its regulatory effect on soil microbiome (Fig. 4). For instance, in the glasshouse experiment the potential nitrogen-fixing activity (the ability of soil microorganisms to fix nitrogen) in the rhizosphere increased by 12-66%in the beginning of the growing season. The actual nitrogen-fixing activity(nitrogen fixation of the specific experimental conditions) increased as well. Usually, denitrification actively occurs in the crops' rhizosphere, leading to the loss of available nitrogen from soil. It is denitrification that is, in most cases, responsible for the low efficiency of nitrogen fertilizers. Studies show that uniquely, the use of Albit correlates with the suppression of denitrification, both potential and actual, in the first half of the growing season (Fig. 4).
Fig. 4. The effect of Albit treatment on the
activity of the nitrogen cycle processes in the rhizosphere of barley in
a pot experiment (Department of Pedology, Moscow State University, 1999).
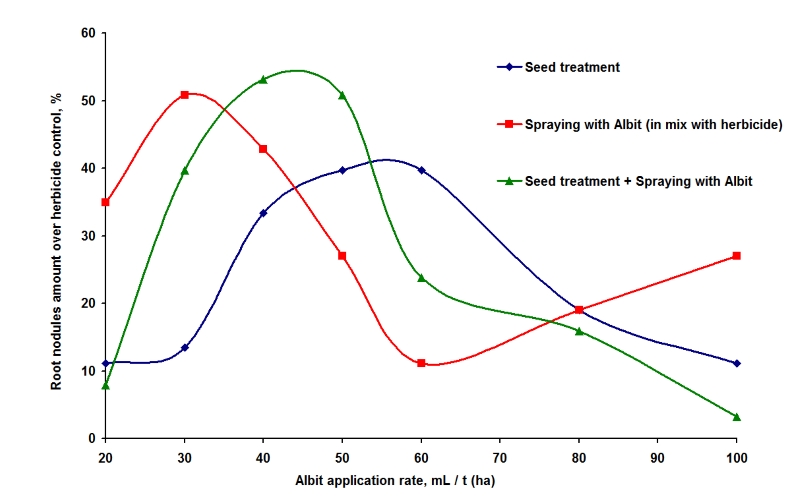
As a result, reserves of available nitrogen in the soil are increased. This effect was confirmed in field trial. In spring of 2019, Albit (1 L/ha) was applied in soil before sowing of white mustard seeds (Tesnitskoye LLC, Aleksinsky district, Tula region). In variant with Albit, amount of available ammonium nitrogen in the arable horizon of soil increased by 22.2% (from 1.8 to 2.2 mg/kg of soil). Sampling was conducted on June 4 in the first half of the growing season. Increase of seed yield was 16.7% to control after Albit application into soil. In the fall, additional carry-over of nitrogen from the crop was observed. However, total nitrogen content in arable horizon was increased because of enhanced nitrogen fixation. According to data of agrochemical analysis, total nitrogen content after harvesting was 1500 mg/kg on the control field and 1700 mg/kg on the field with Albit (nitrogen reserves in the soil increased by 13.3%). Influence of Albit on soil N2-fixers was evaluated on the most active ones, Rhizobia. Indigenous soil population of Rhizobia is usually insufficient for formation of required amount of root nodules of legumes. Generally, additional treatment of seeds with Rhizobia inoculants is used to overcome this problem. Albit allows to use the alternative approach directly in the field: stimulation of activity and virulence of natural soil Rhizobial population. In field trial, performed by National Institute of Biological Plant Protection (Rus. Acad. Agric. Sci.) (Krasnodar, 2010) on soybean, it was shown, that Albit improves formation of root nodules on non-inoculated plants. In the field trial, application of Albit (seed treatment and spraying in combination with herbicides 30-50 mL/t) increased amount of nitrogen fixing nodules per one plant up to 13.5-53.2% over herbicide-only control. Seed treatment with Albit increased the amount of nodules up to 39.7% over control, sprayings with Albit in combination with herbicide – up to 50.8%, combined application (seed treatment + spraying) – 53.2% (Fig. 5). Increased amount of nodules proportionally resulted in increased crop yield (up to 17 % over control).
Fig. 5. Influence of application rate of Albit and different ways of treatment, on the amount of nitrogen fixing nodules on soybean roots (field trial by National Institute of Biological Plant Protection, Krasnodar, 2010)
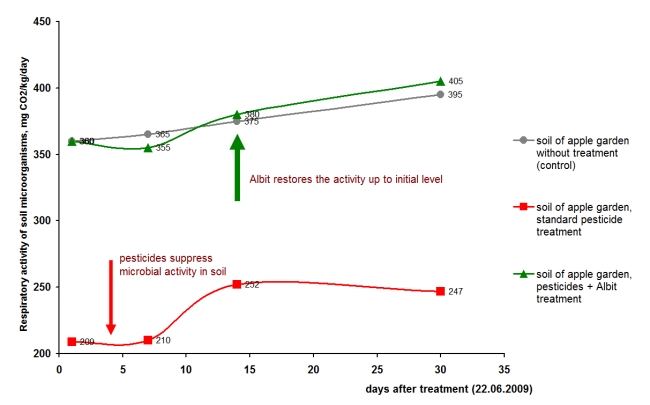
At the basis of the action of Albit on the soil microbial community, in our opinion, lies the properties of poly-beta-hydroxybutyric acid (see details). This compound, like many polymers of biological origin (starch, cellulose, chitin) promotes initiation of microbial succession, formation of specific hydrolytic, and related organisms community, which has an indirect positive effect on plants. As a result, the Albit application provides an additional input of nitrogen and other nutrients in plants (see more details). Chemicalization in agriculture using intensive technology destroys the natural microbiocenosis capable of protecting plants from phytopathogens. Pesticides inhibit microbial activity of the soil by 30-50% [Karpun, Janushevskaya, 2014]. In perennial field trials performed at National Scientific Research Institute of Floriculture and Subtropical Crops RAAS (Sochi) it was shown that Albit is able to reduce the negative impact of pesticides (based on dithianon, α-cypermethrin, λ-cyhalothrin, δ-methrin) on soil microbial consortium [Janushewskaya, Kaprun, 2011]. Albit increases the resistance of soil microflora to the toxins, and normalizes its biological activity which is suppressed by the usage of pesticides (Fig. 6). Field trials were carried out on plantations of peach and apple trees (the farm gardens of All-Russian Scientific Research Institute of Floriculture and Subtropical Crops RAAS (Sochi)). Albit was used within standard protection protocol of gardens in conjunction with chemical pesticides (insecticides and fungicides). The first treatment of peach trees with Albit and dithianon was performed before flowering stage. Albit was used together with pyrethroids after flowering during the second and third treatments. Gardens without usage of pesticides, as well as virgin forest were taken as a control. During the field trials the dynamics of the overall biological activity of the soil all over the growing season was determined.
Fig. 6. The dynamics of potential activity of soil of apple garden after treatment with insecticide (a.i. is δ-methrin) in conjunction with Albit
The treatment of plot trials of apple garden with chemical pesticides decreased the basal respiratory activity of soil microflora (Fig. 7). Complete normalization of the respiratory activity of the soil was not observed even a month after application of pesticides. Albit used together with pesticides significantly reduced their negative side-effects: addition of Albit to standard chemical treatment almost regained the level of microbial activity on the level of undisturbed soil. These patterns were observed annually during the all studied period of 2008-2010 both with insecticides, and fungicides). The adaptogenic activity of Albit was especially expressed in drought conditions of 2009. It was found that the intensity of the adaptogenic properties of Albit essentially depends on the soil conditions, which stimulate metabolic processes. The main non-specific mechanism of adaptogenic action of Albit is the activation of substrate-induced aerobic respiration, which laid in the base of increasing intracellular bioenergy resources and provide adaptation of microbial consortium to stress factors of different origins. Albit is able to significantly reduce oil pollution of soil by stimulation the natural soil microflora and plant growth. The rate of oil decomposition in the soil increases in average by 1.67-3.15 times under the influence of Albit. Industrial tests showed that Albit together with sowing of oil-tolerant grasses reduces oil contamination of soils by 1.5-10.0 times during one growing season.
Fig. 7. Visual effect of Albit action on soil microflora. After application of Albit as a biomeliorant, rapid development of mycorrhiza in infertile clayey soil of construction dumps was observed (territory of Albit LLC production facility, Moscow oblast, 2016)
Thus, Albit exerts beneficial influence upon plants, increases their mineral nutrition, reduces the possibility of pathogens injury, reduces the toxicity of soils, acting indirectly through the soil microbial community. In this case, Albit acts as a biomeliorant and bioremediant of soils. This direction of Albit activity and also immunizing and anti-stress activity supply guaranteed positive effect of Albit application. Albit acts as an integrated, balanced, and protective bio-stimulant, embracing nearly all spheres of plants vital activity.
Literature
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Terms and Conditions
|
|